A Global Health & Research Company Partner
Let’s Accelerate the Future of Health Together

Future of Medicine health screenings
Make your studies available to Future of Medicine members

Research Sites across the globe
We establish and conduct high-quality research operations wherever they are needed

Site Support Solutions
Save site time and resources by leveraging our Central Clinical Services or Site Staffing solutions
What We Do
1
Community Health Screening

We conduct large-scale community-based health screenings and education that streamline access to clinical research studies.
- Ongoing pre-screening activity
- Independent of study protocols
- Blood Tests and Medical Records
- Sites can start enrolling at study activation
- Multiple therapeutic areas
- Deep-rooted community partnerships
- Referrals that are interested in participating
2
Clinical Research Sites and Site Support Services

Our global network of sites and PIs provide a wide range of research services.
- Care Access Global Site Network
- Virtual PIs and regional sites
- Mobile research capabilities and infrastructure
- Centralized Referral Management
- Centrally Managed Long Term Study Follow-up
- Central Visit Management
- Specialized on site staff augmentation
- Start-up and Close-out support
- Training programs
Active Studies


























































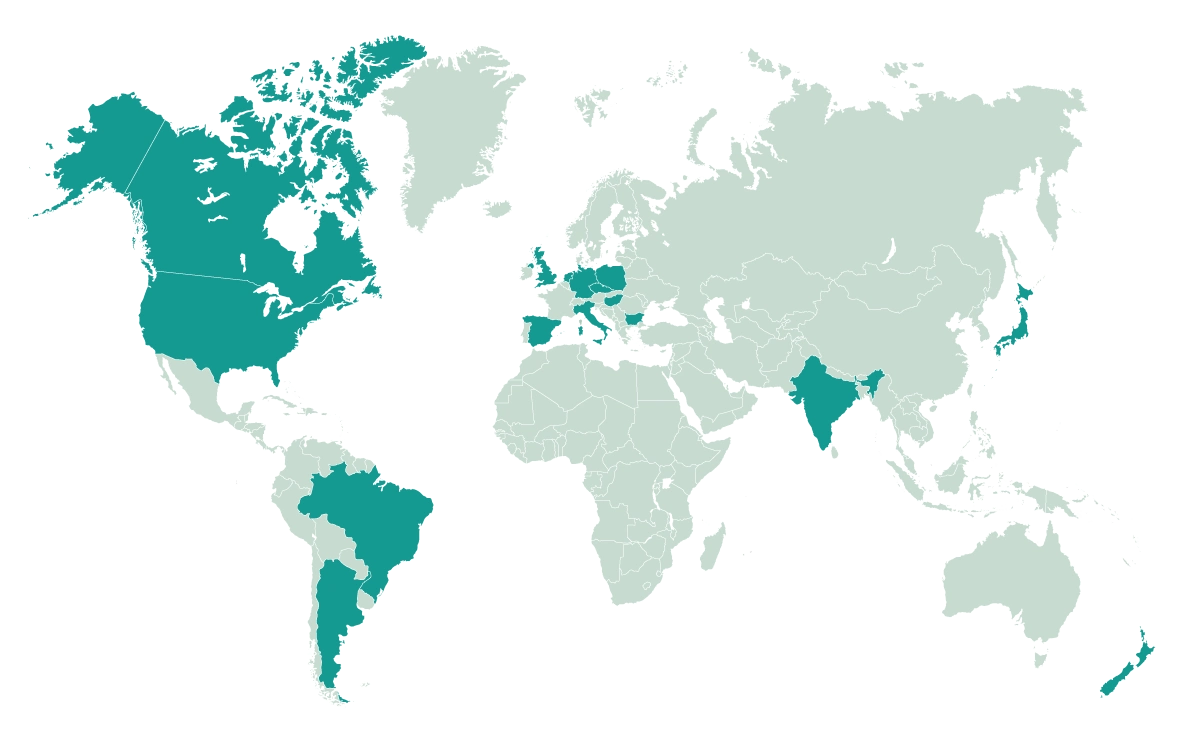
Our Reach
We operate in 12 countries countries including the United States, Canada, Brazil, and Poland.
200+
Permanent Site Locations
250+
Investigators
150+
Mobile Health Clinics
~450
Clinical Research Professionals
300+
Clinical Trials
14
Therapeutic Areas
150,000+
Participants screened per year
~20M
Patient Network
Therapeutic Areas
 Cardiology
Cardiology Dermatology
Dermatology Endocrinology
Endocrinology Family Practice
Family Practice Gastroenterology
Gastroenterology Gynecology
Gynecology Infectious Disease
Infectious Disease Internal Medicine
Internal Medicine Neurology / CNS
Neurology / CNSMetabolic Disorders
Nephrology
 Psychiatry
Psychiatry Respiratory
Respiratory Rheumatology
Rheumatology Vaccines
Vaccines
Centralized Research Staff to Support
- Patient referral management
- Long term follow-up visits
- Remote data entry
- Psychometric assessments
- Remote Medical oversight and safety reviews
- Medical data review
- Audit readiness, preparation, and support
- And more….
Our Journey
Redefining What’s Possible in Clinical Research
Throughout our history, Care Access has consistently developed novel solutions to major constraints in the traditional clinical trial model. Now, we’ve integrated our proven solutions and practical experience into a leading-edge delivery model that accelerates study recruitment times while improving access to research in more communities.
See how we’re evolving the way that clinical trials are conducted to match the rapid pace of today's medical discoveries.
Scientific Publication
Peer-Reviewed Study Shows How the Unique Care Access Community-Based Site Model Improves Trial Enrollment and Efficiency
A 2025 study published in the peer-reviewed medical journal Frontiers in Medicine shows how Care Access’s mobile and community-based approach is transforming the clinical trial process by:
- Improving recruitment efficiency by making research more accessible
- Delivering higher randomization rates
- Giving underrepresented communities a chance to take part
- Maintaining strong participant retention
New PI Development
Phase
2
Gastroenterology
~5 new sites
Created a system for enabling new-to-research physicians to become PIs with 360° support from experienced staff, helping address the industry-wide PI shortage.
Rapid New PI Development
Phase
2
Immunology
~20 new sites
Scaled our PI development capability to rapidly create new research sites and accelerate enrollment for a specific study that had few feasible sites.
Multi-Site PI
Phase
3
Home Infusions
~50 new sites
Developed the model for remote PI oversight, which reduces the burden on on-site sub-investigators and enables research studies to be offered in more locations.
At-Home IP Dosing
Phase
3
Infectious Disease
~5 new sites
Built leading-edge mobile infrastructure and logistics to precisely manage investigational products anywhere, even in places where research infrastructure doesn’t yet exist.
Centralized Virtual Staff
Phase
3
Neurology
100+ new sites
Implemented a remote central team to efficiently manage study tasks, allowing local site staff to focus on work that requires in-person activity.
Pop-Up Mobile Sites
Phase
3
Vaccine
~30 new sites
Combined PI development, multi-site PI, advanced IP management, centralized virtual staff, and mobile infrastructure to rapidly build mobile sites in areas with very limited research infrastructure.
Pop-Up In-Clinic Sites
Phase
3
Pulmonary
~30 new sites
Extended our pop-up mobile site model to medical clinics, making study participation possible for communities underserved by clinical research.
Split Screening & Enrollment
Phase
3
Cardiometabolic
~60 new sites
Designed and implemented a study-agnostic pre-screening program that offloads pre-screening work from research sites, so they can begin enrolling participants immediately when studies are activated.
Presentation
Adoptability as the Necessary Criteria for Impactful Innovation

We support sponsors across their portfolios. For the highest-priority studies, we bring unique solutions.

Super Site Capabilities
Care Access further supports the industry with Super Site Capabilities that augment the traditional research site.
Permanent Site Locations
- Traditional Research Sites
- State-of-the-Art Facilities
- Long-Term Community Connections
- Function as Site Network Logistics Hubs
Centralized Clinical Operations
- Quality System
- Virtual PIs when valuable
- Study Management
- Site Orchestration
- Control Tower
Mobile Research Site Infrastructure
- Mobile Clinical Research Vehicles, Trailers
- Traveling Sub-Investigators
- Traveling Clinical Research Staff
- IP Management & Transport
Patient Centric / Patient Convenience
- Patient Education
- High-Capacity Call Center
- Virtual Coordinators
Central Logistics
- Central Labs
- Distribution Hubs
- Fleet Shop
Grassroots Community Engagement
Our Commitment to Communities
We work with hundreds of communities each year to deliver health services and research where it is most needed.
Through our Future of Medicine program, we provide our members advanced health screenings, information about research studies, and other education and services.
And through our Difference Makers program, we support and amplify the impact of local leaders and organizations that work to improve their communities’ health and wellness.
Communities Embracing New Access to Research


Lima, OH: "A Privilege to Have Access"
Watch Video

Mesa, AZ: "Right Here in Town"
Watch Video

Hope Mills, NC: "Finally Included in Something"
Watch VideoMaking Space for Clinical Trials in Senior Communities


Retirement Community Embraces Clinical Research
Watch VideoDiversity & Representation
Care Access partners with Sponsors to help achieve their research diversity and representation goals.
We take a grassroots approach to engaging with diverse and under-represented communities. Our Super Site Capabilities make clinical research available to those communities even when there is no existing clinical trials infrastructure.
Therapeutic Area Focus
Building Therapeutic Area Ecosystems.
Care Access hyper-focuses on a set of therapeutic areas that are high-priority for the industry and builds capabilities that strengthen our ability to support those studies. This includes: study-agnostic screening, scientific expertise via advisory board, and community awareness building around the condition.
Currently, we are building the following Therapeutic Area Ecosystems:
- Alzheimer’s
- Gastroenterology
- Cardiovascular
- Diabetes & Weight Management
- Infectious Disease
Learn more about our Cardiovascular Ecosystem
Collaborating with leading AMCs and HBCUs through American Heart Association (AHA) research network
Lp(a) screenings built around ecosystem of healthcare providers, community leaders, and individual ambassadors
Establishing Care Access’ Cardiology Scientific Advisory Board
Accelerating the Future of Medicine for Everyone
We’re not just providing more patients with access to innovative research studies, we’re making the future of health better for all.
With the support of 14 of the top 15 pharmaceutical and biotech partners, we are helping people learn more about their health, access health resources they need, and participate in research to help find new medicines and cures.
Interested in exploring how we can support your research studies?
Contact Us






